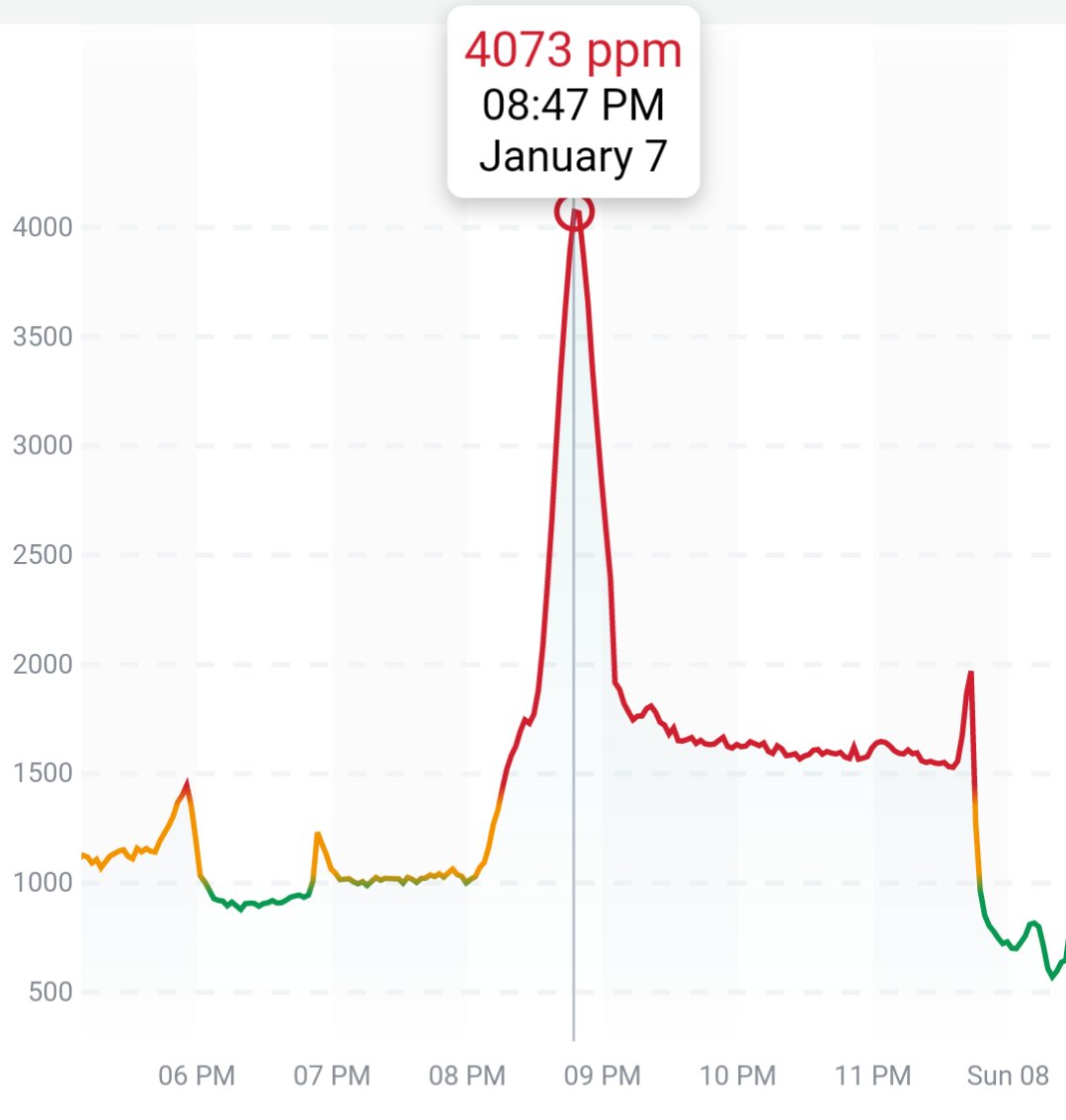
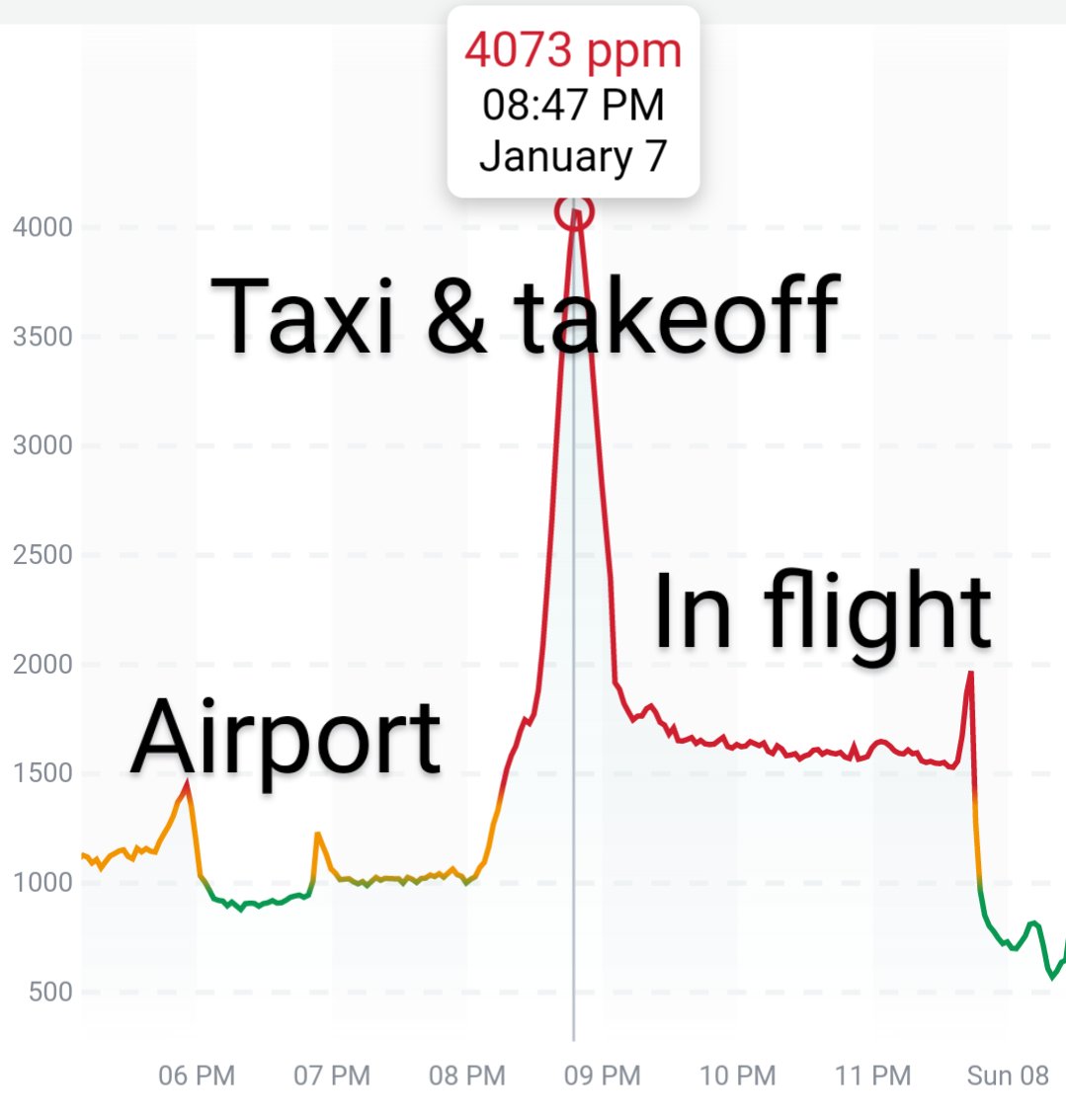
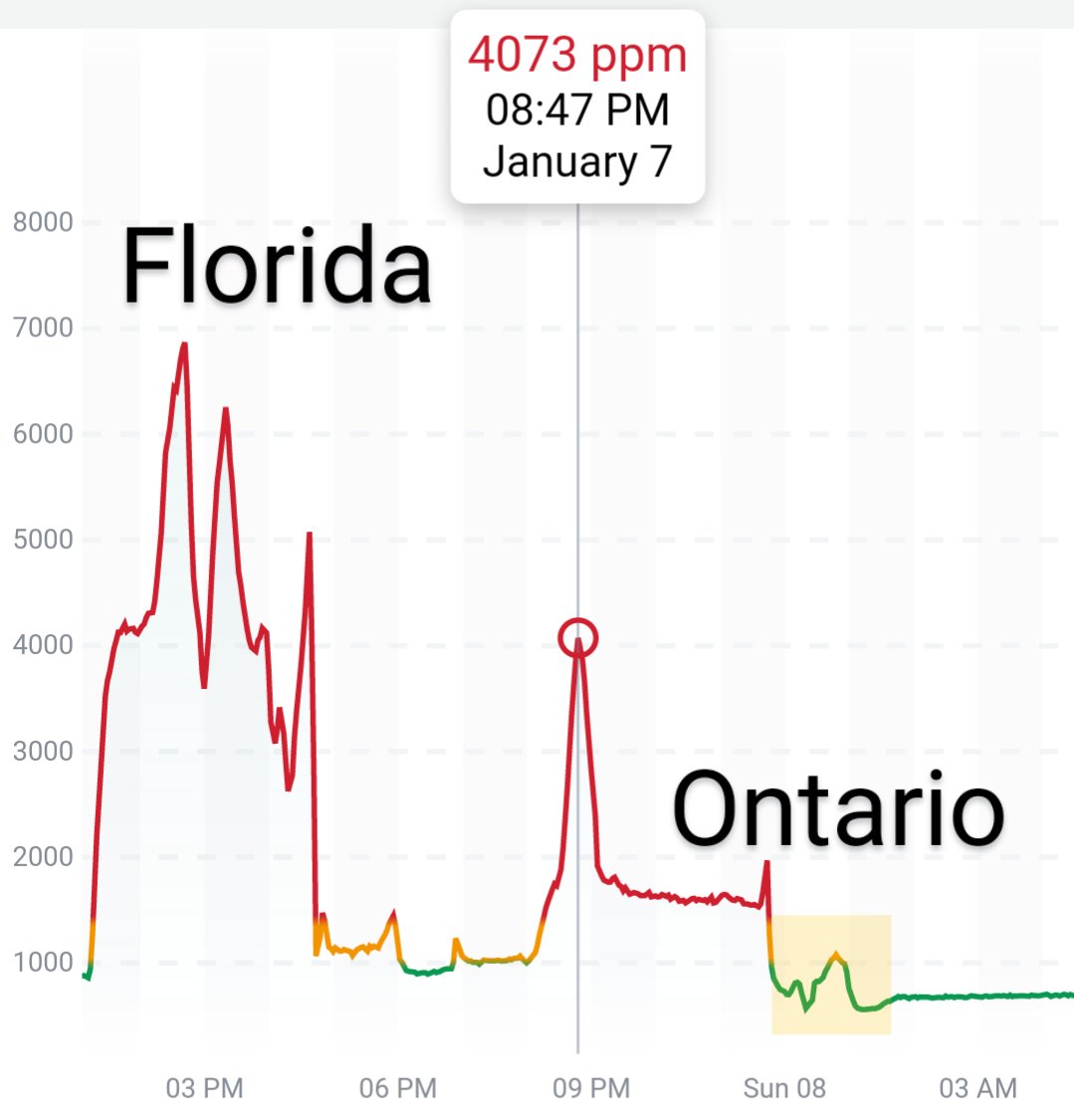
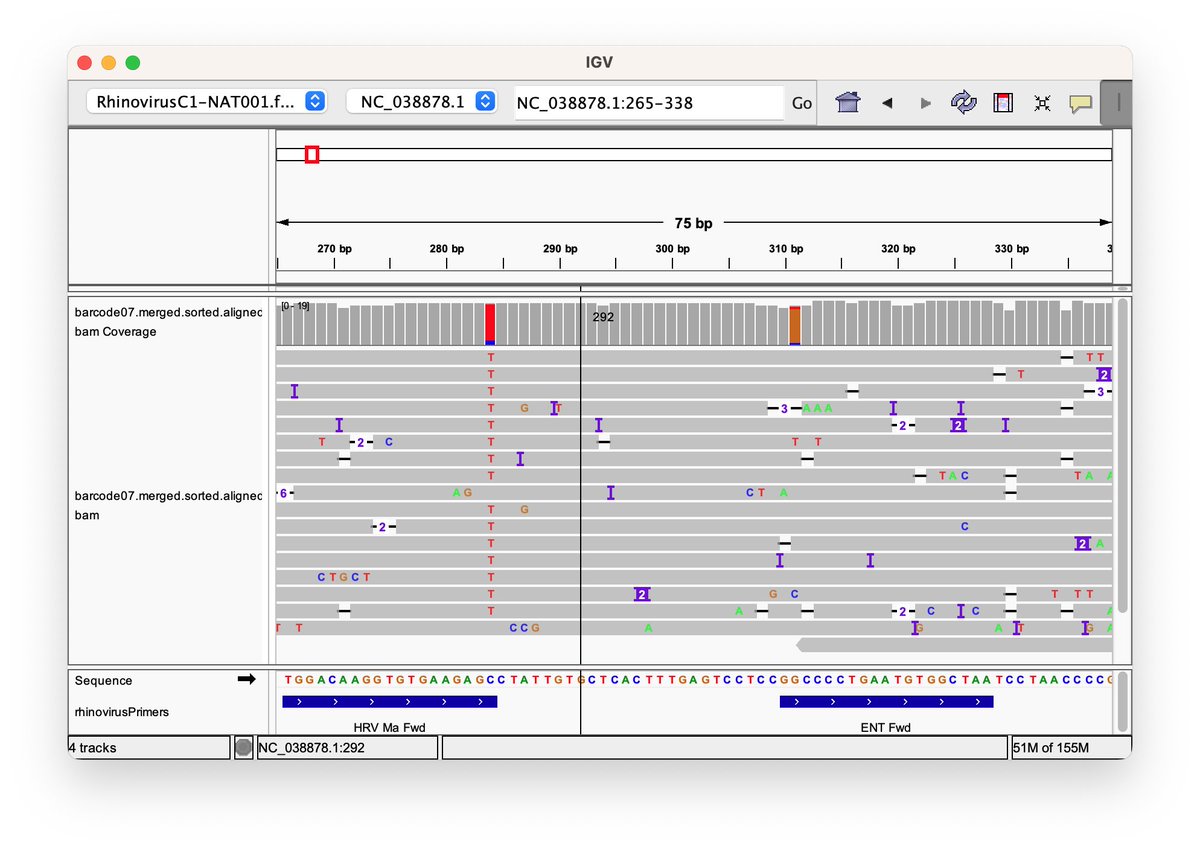
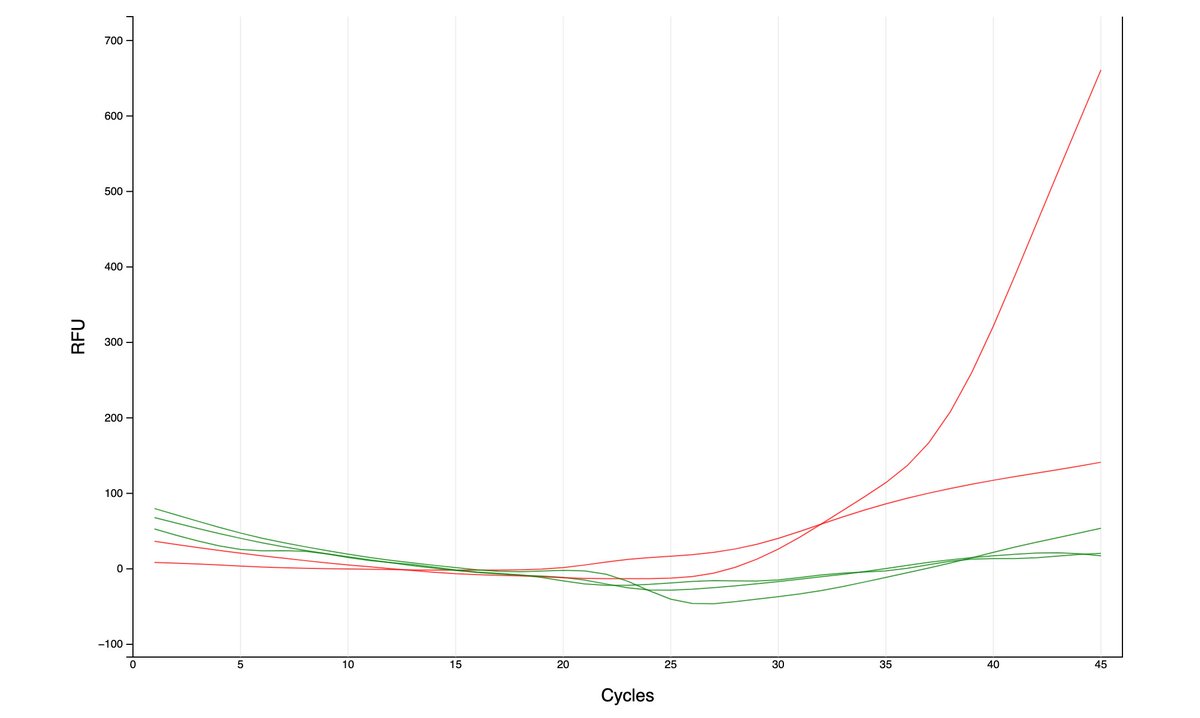
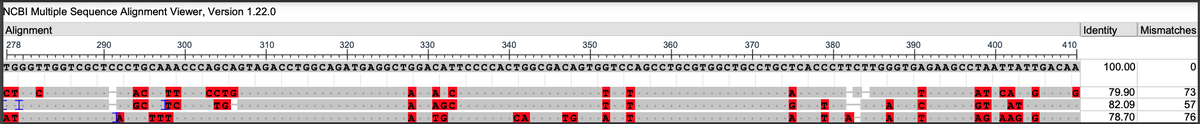
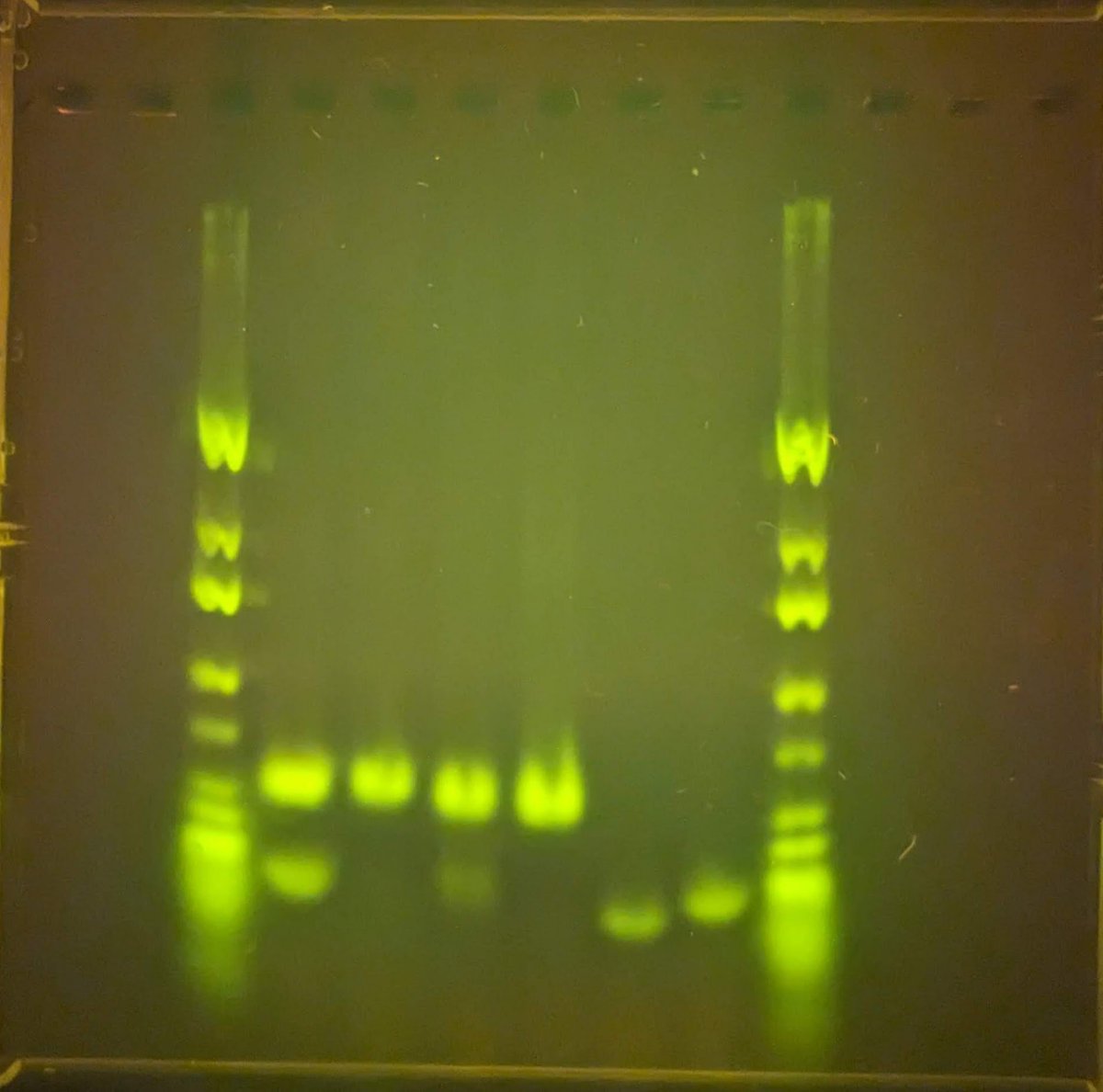
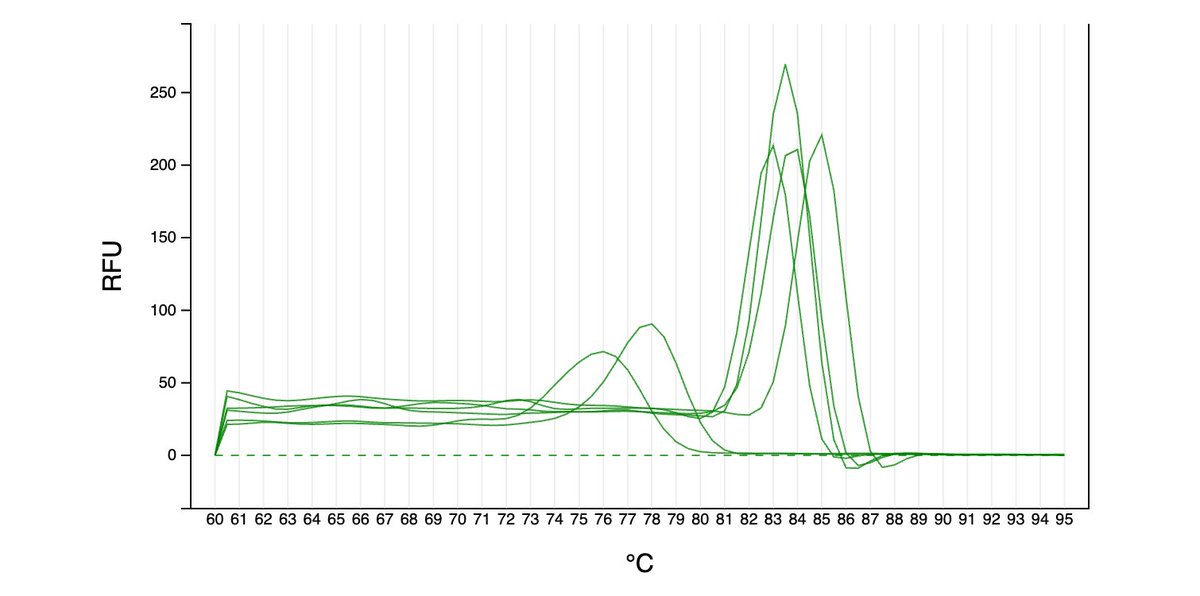
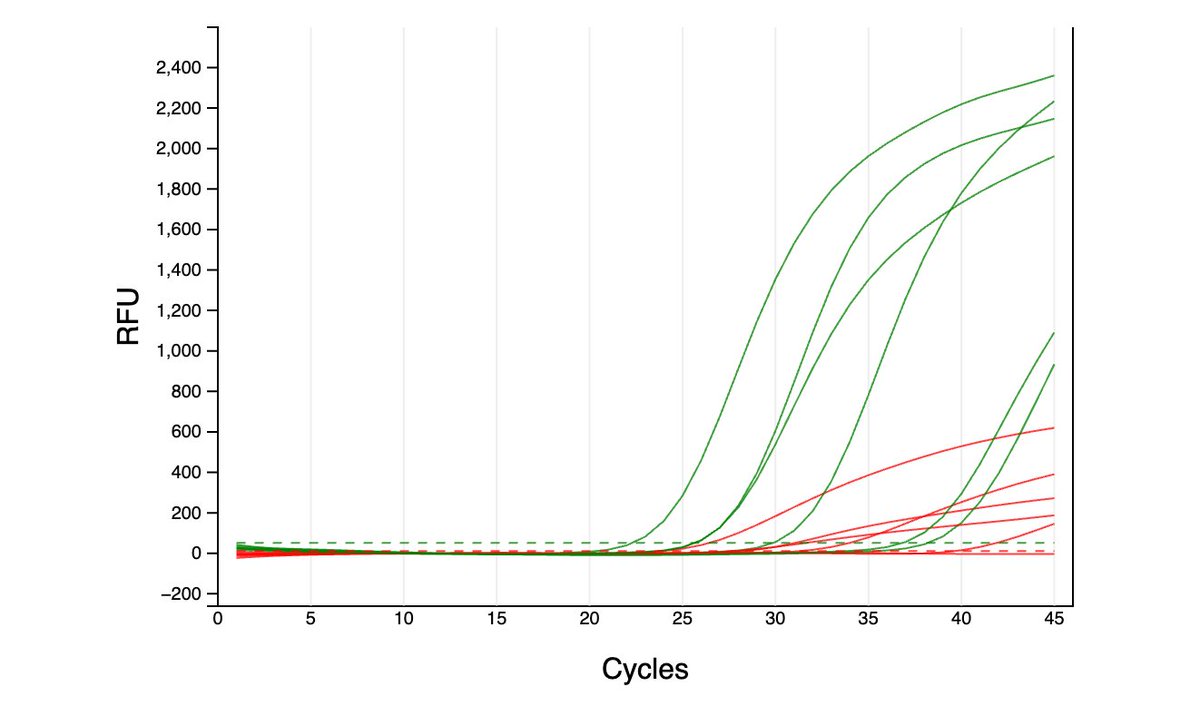
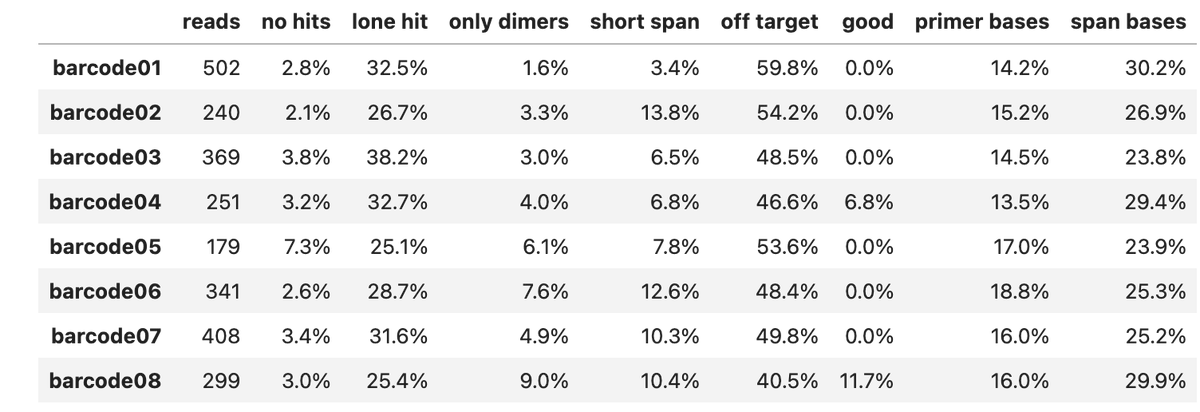
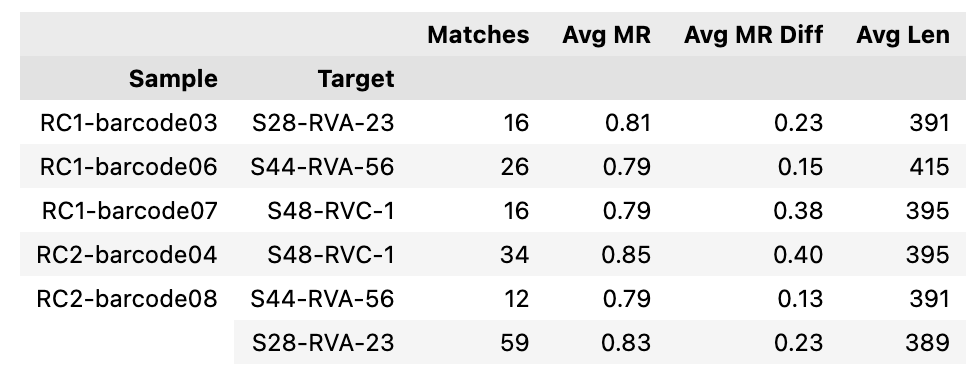
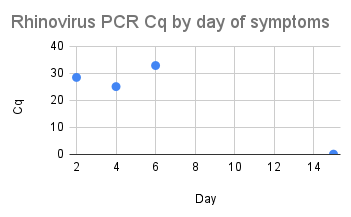
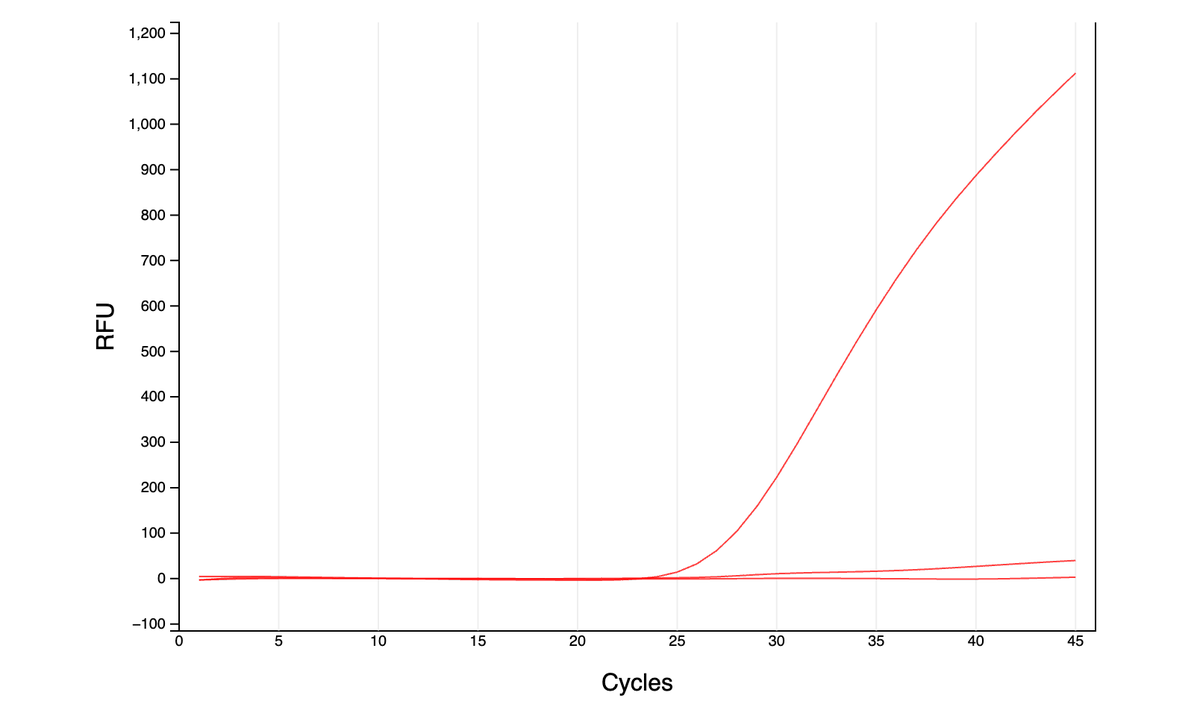
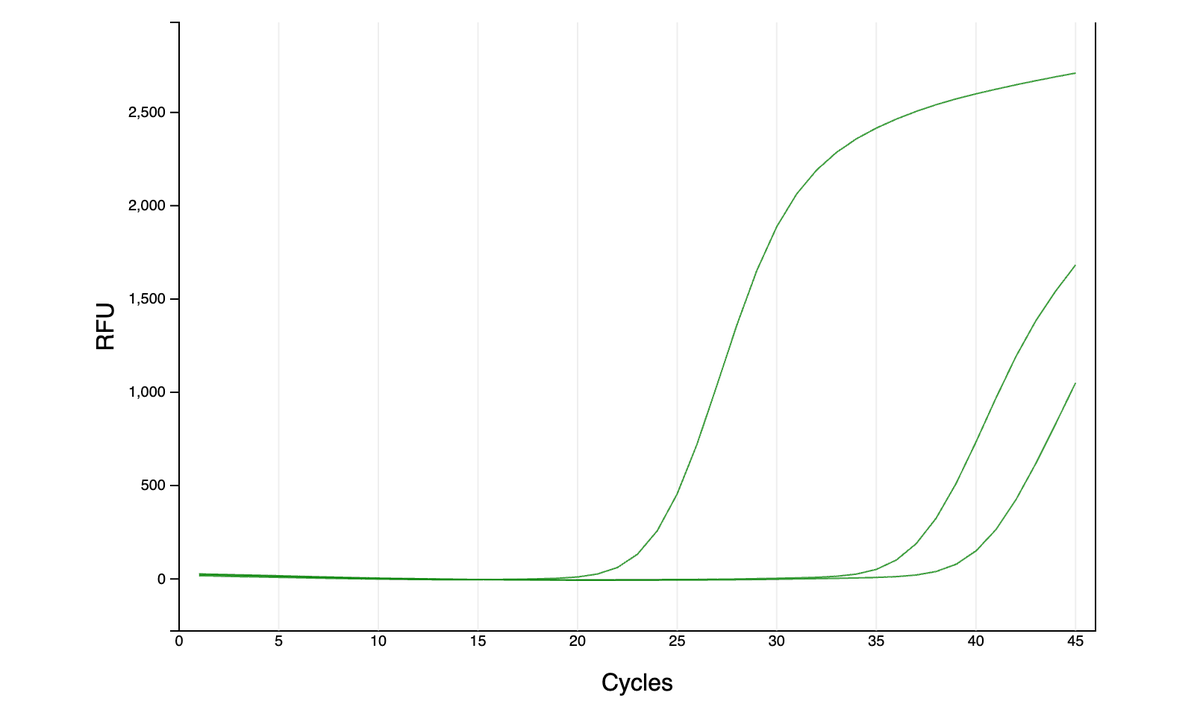
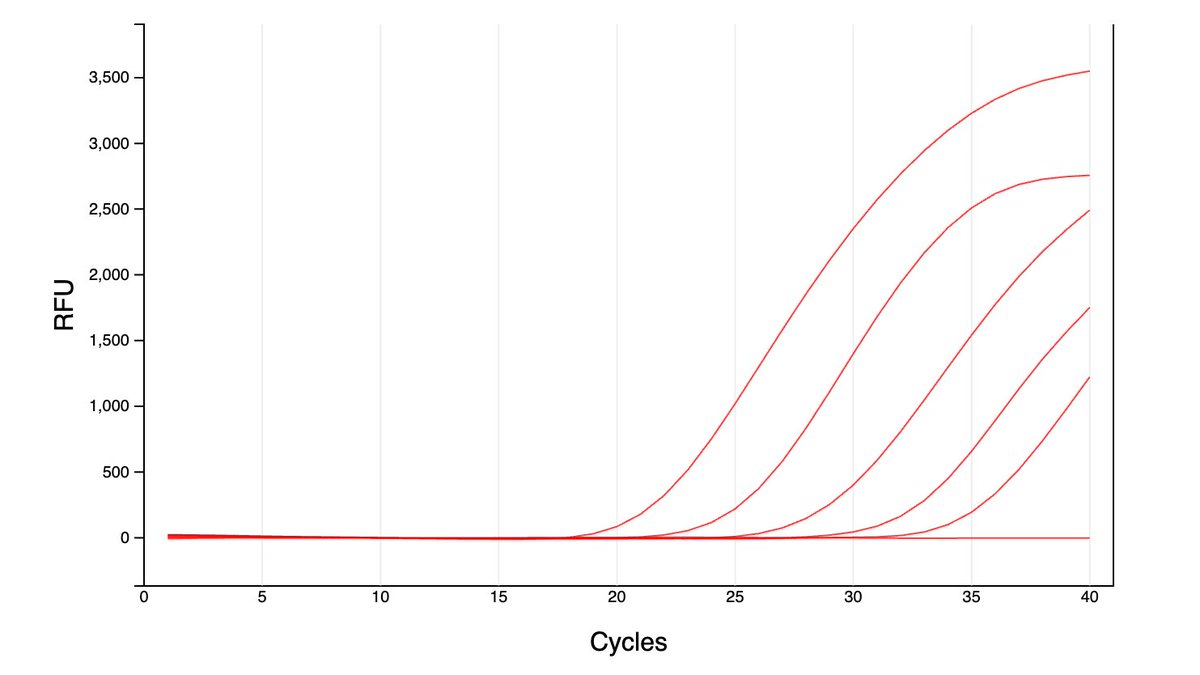
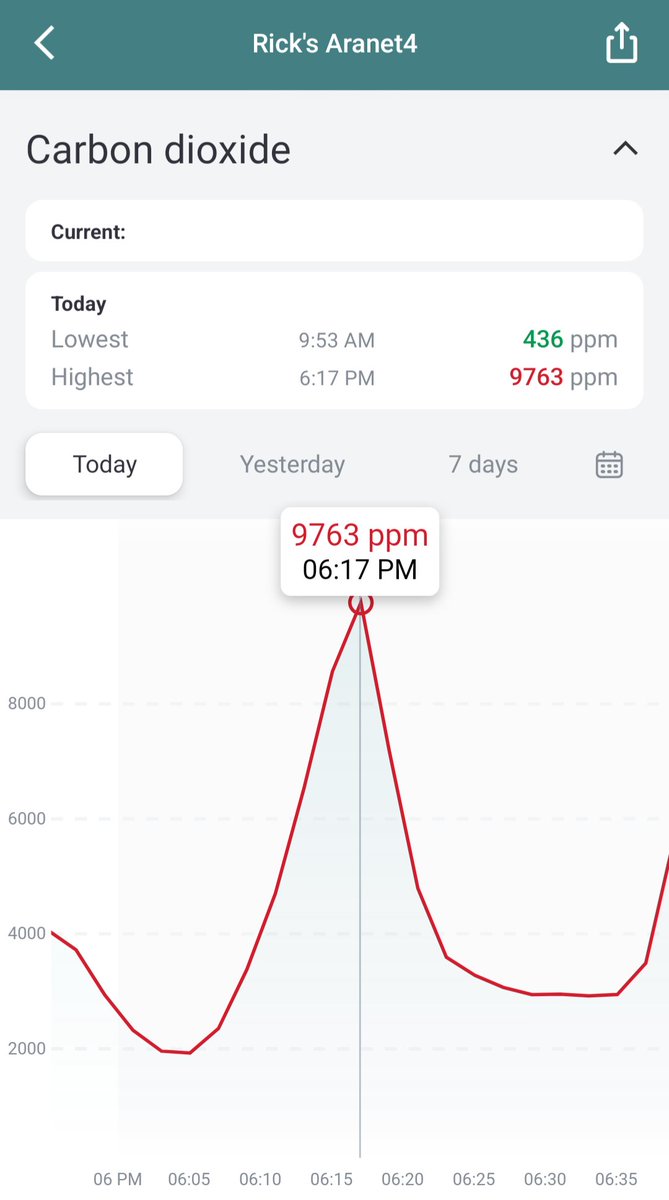
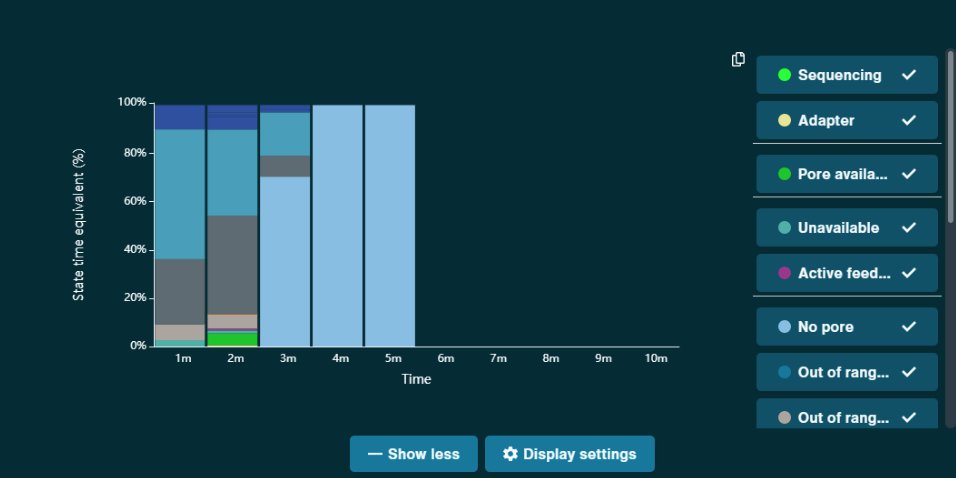
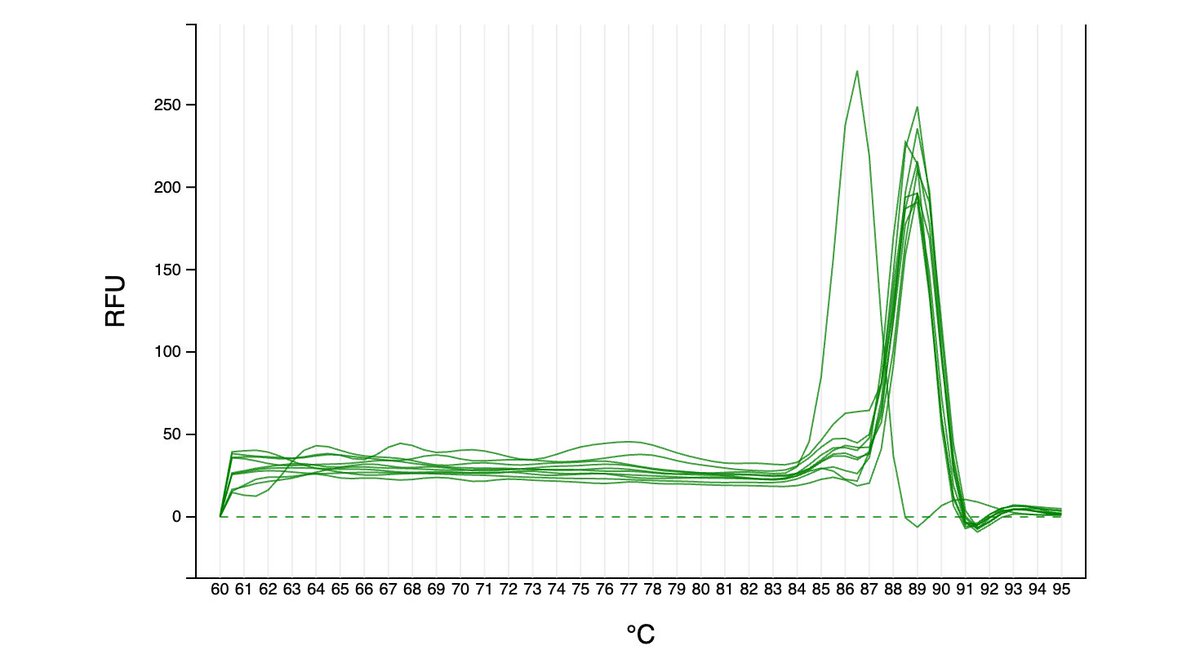
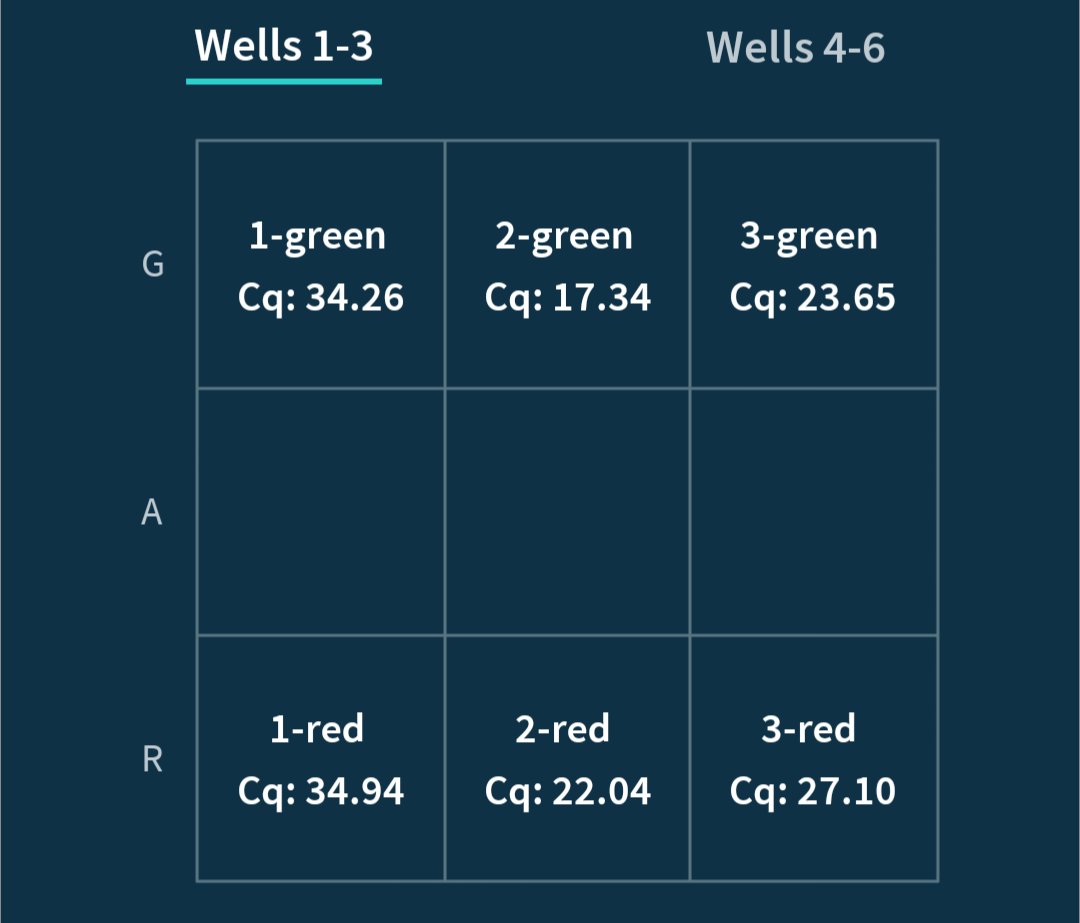
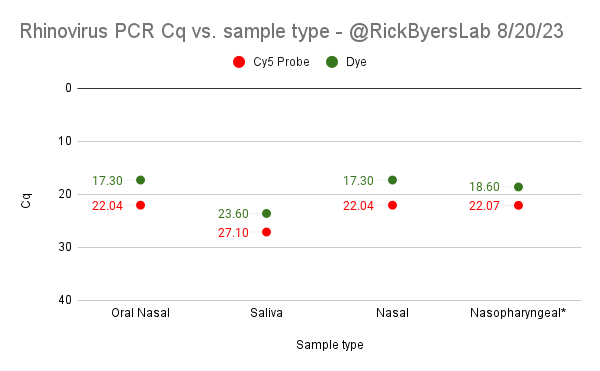
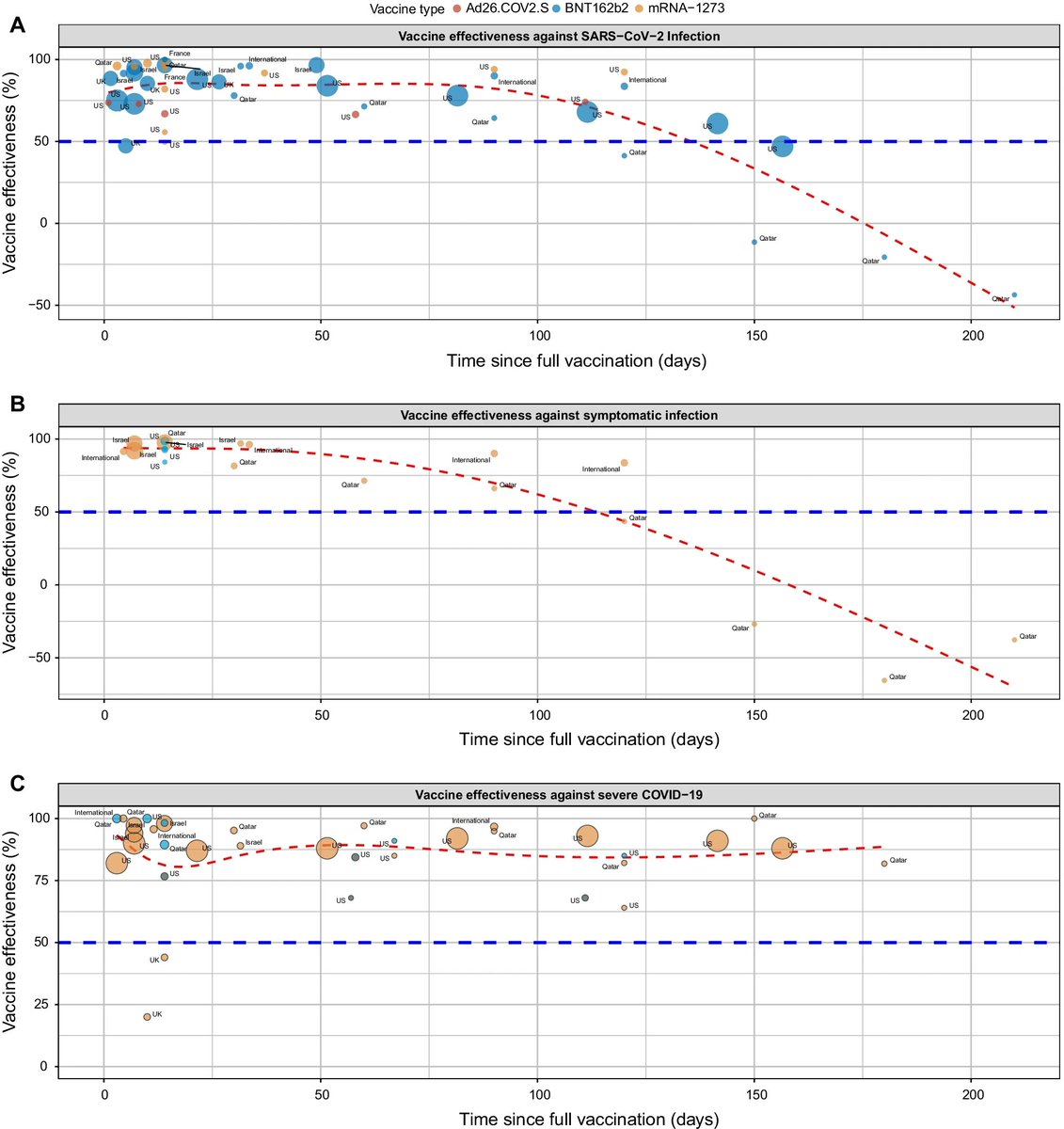
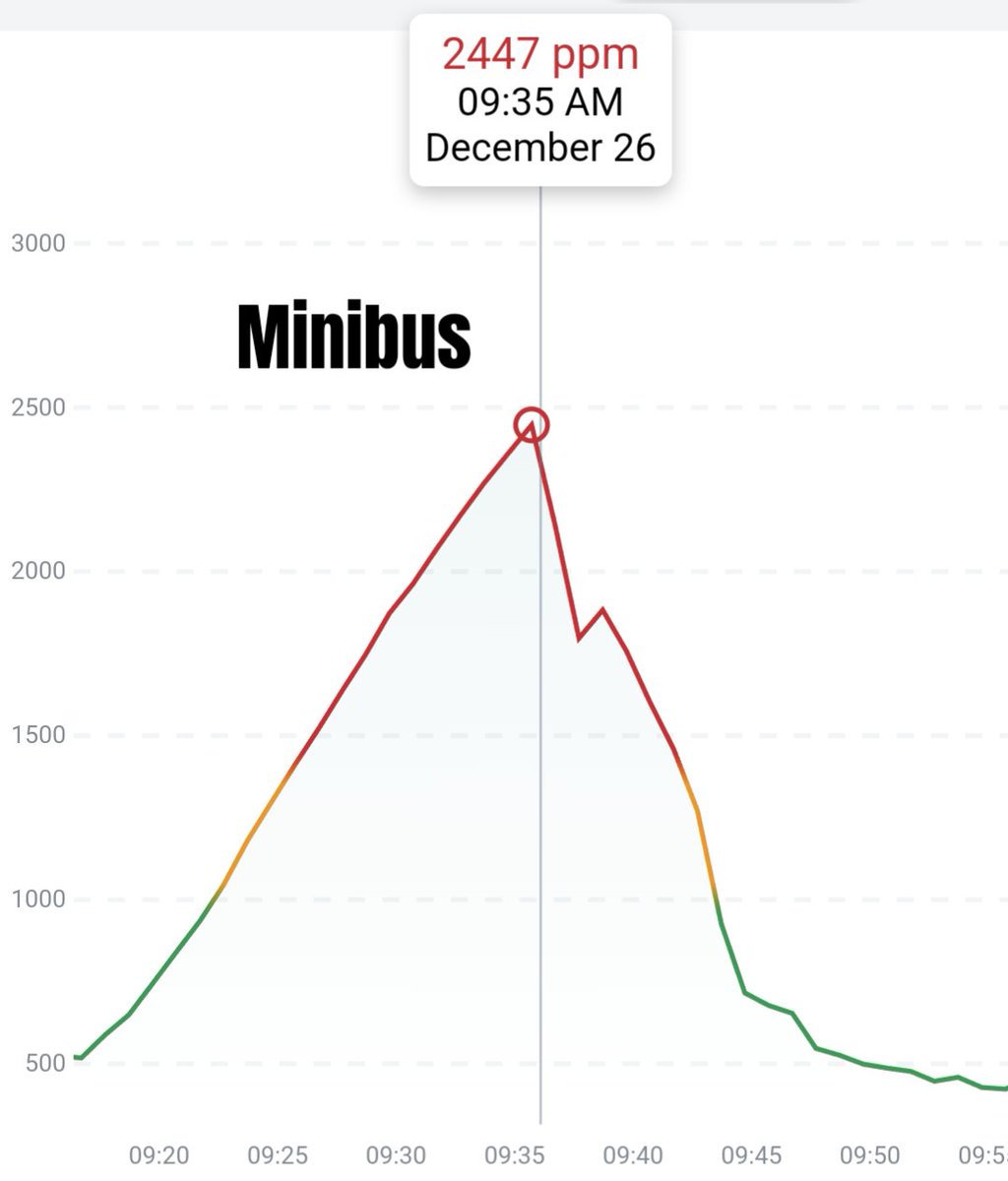
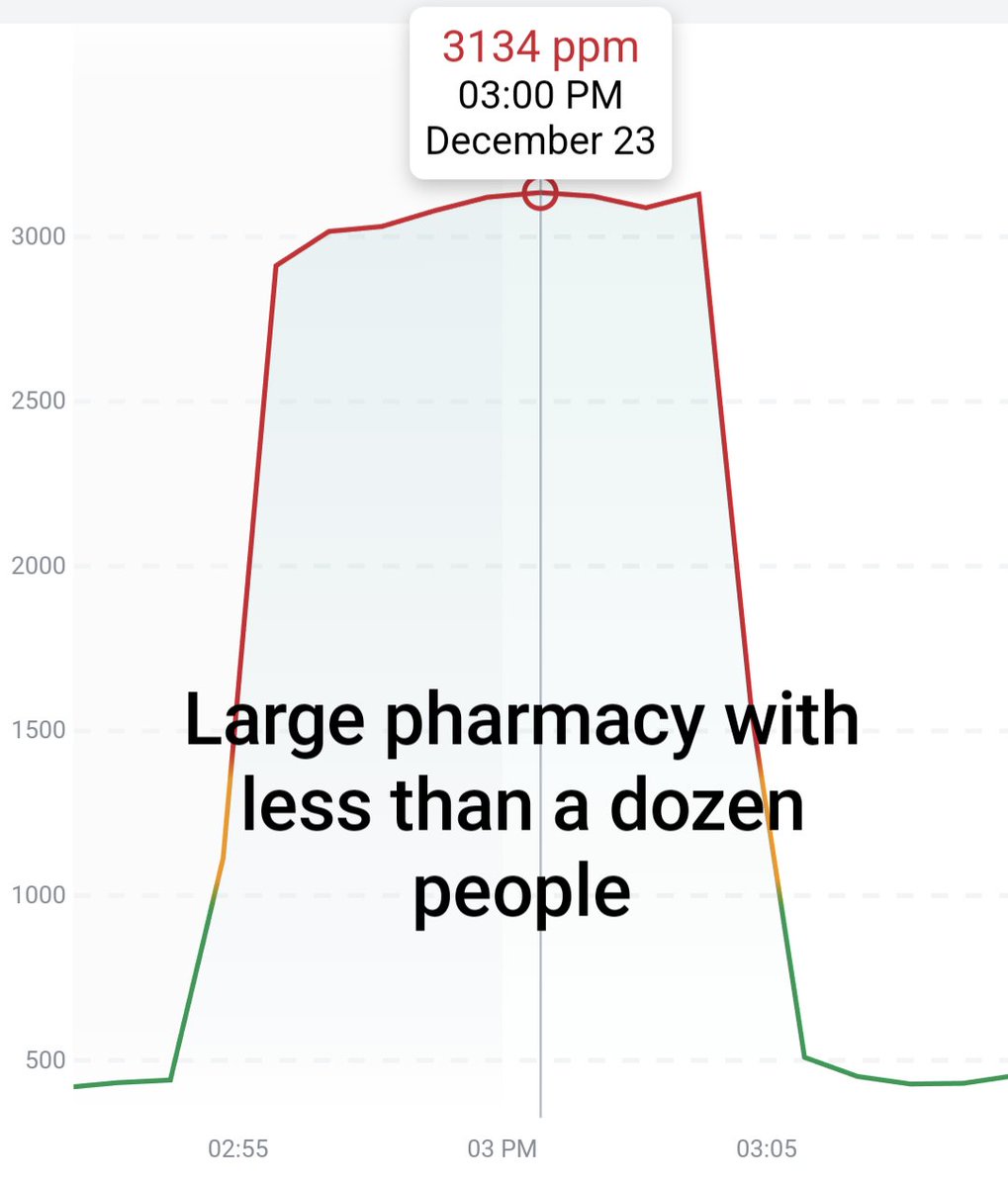
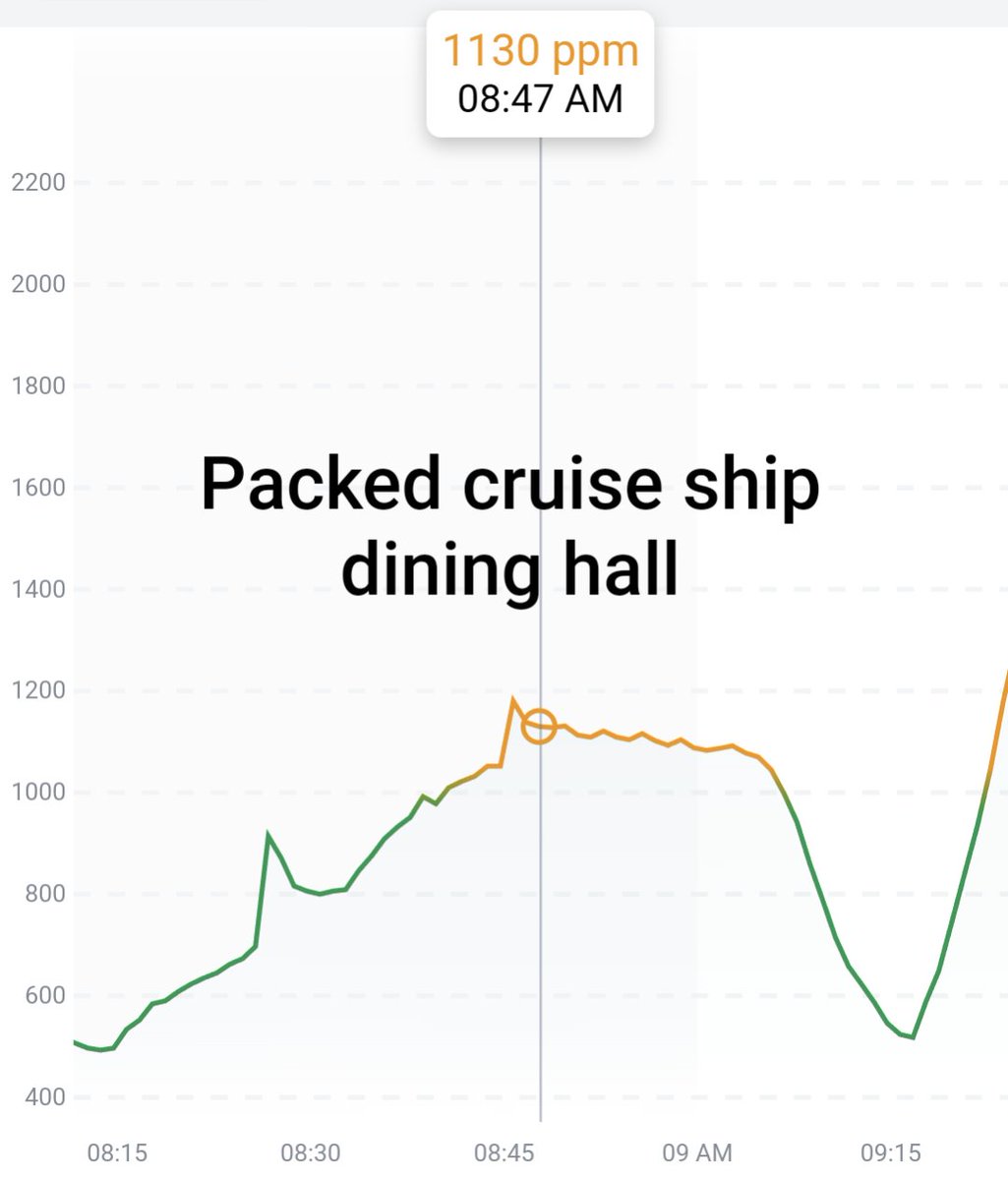
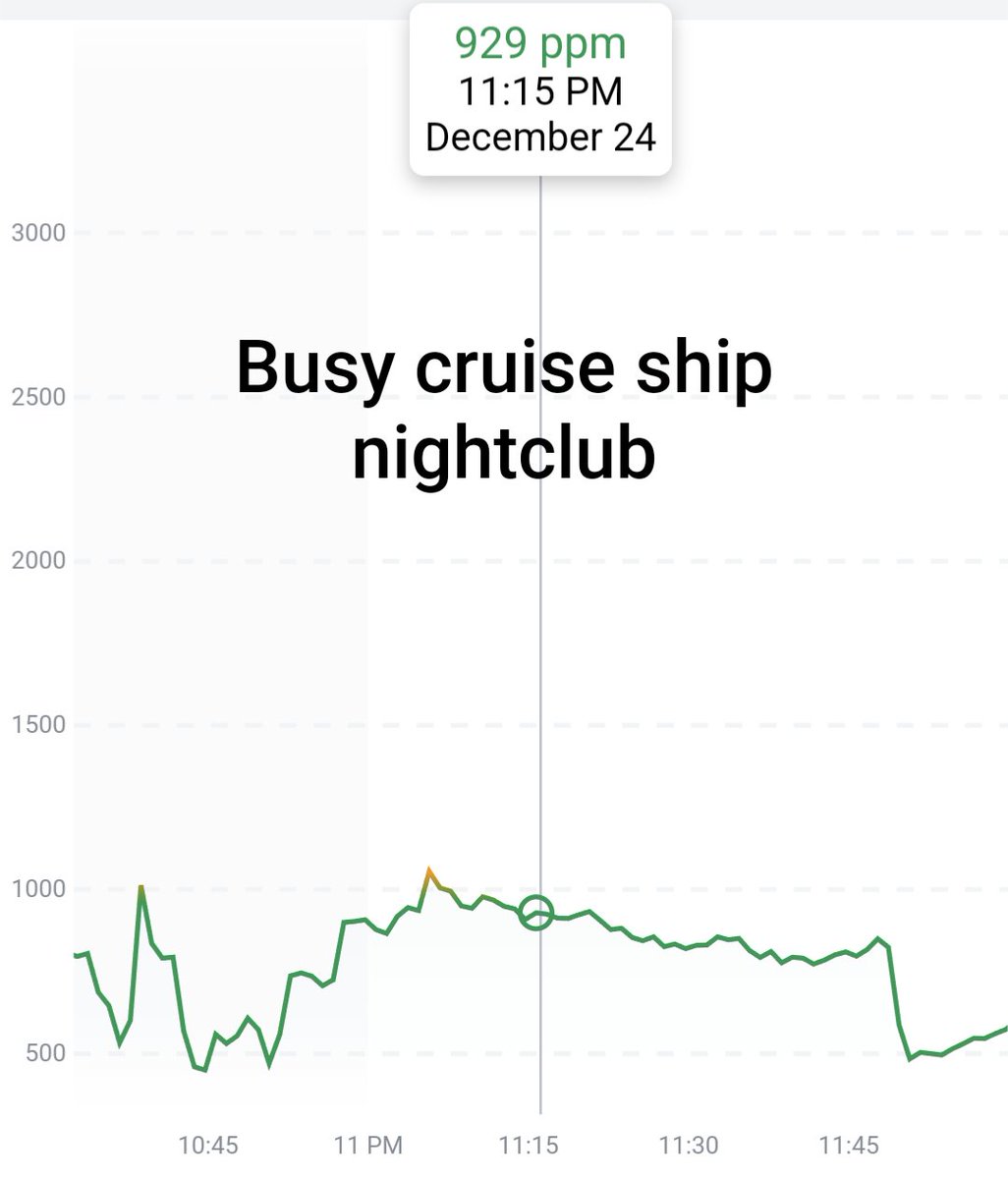
Can you guess what I was doing last night from this CO2 log?
There was a very sick kid in the seat behind my son, coughing and wiping her nose the whole flight. A flight attendant offered the Mom a mask for the child, but they didn’t wear it. I guess we’ll know in a few days if our N95s were good enough to protect us.