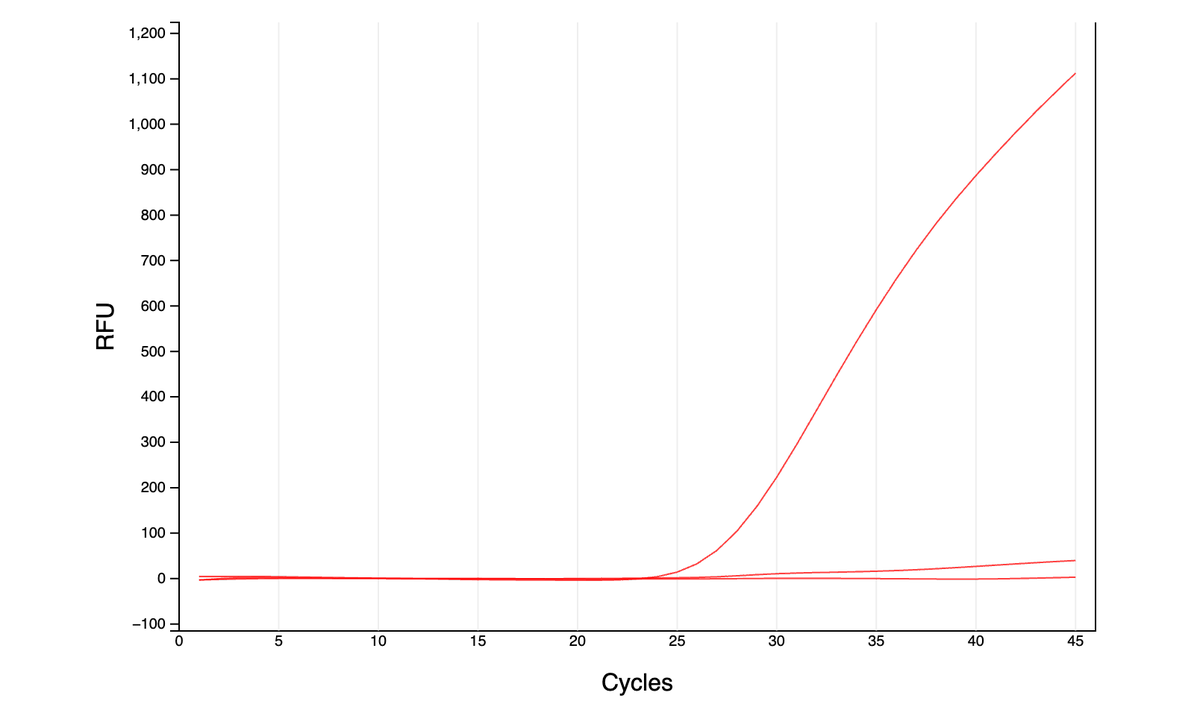
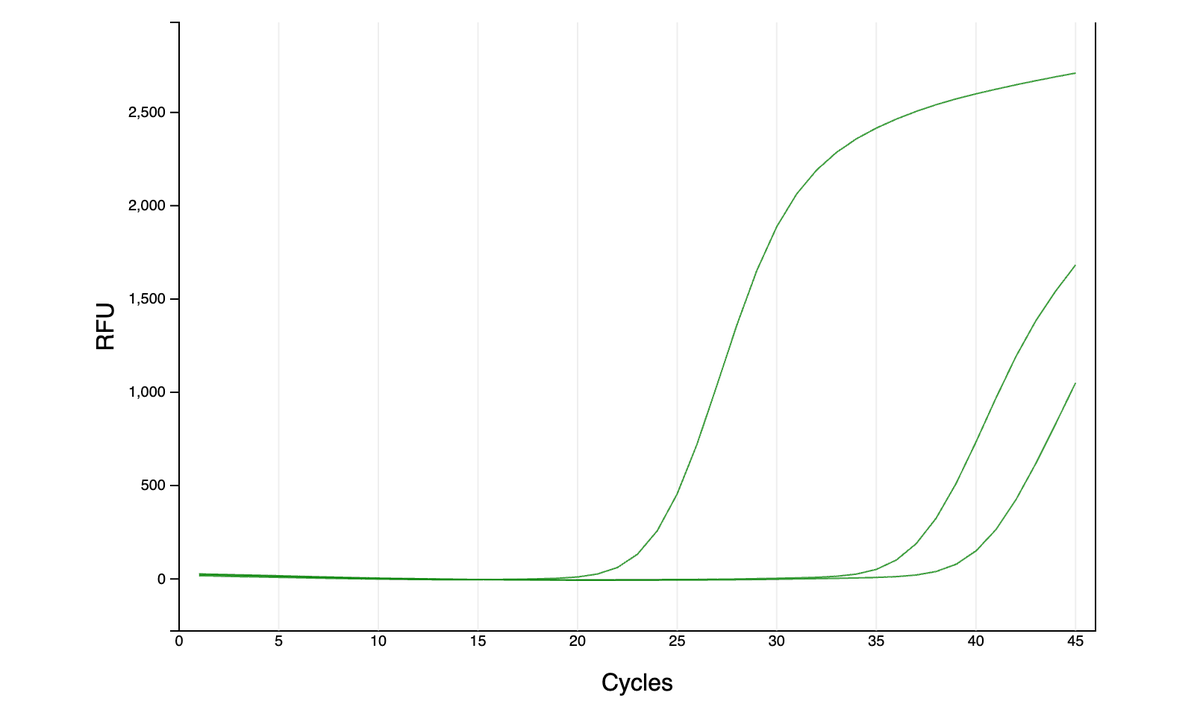
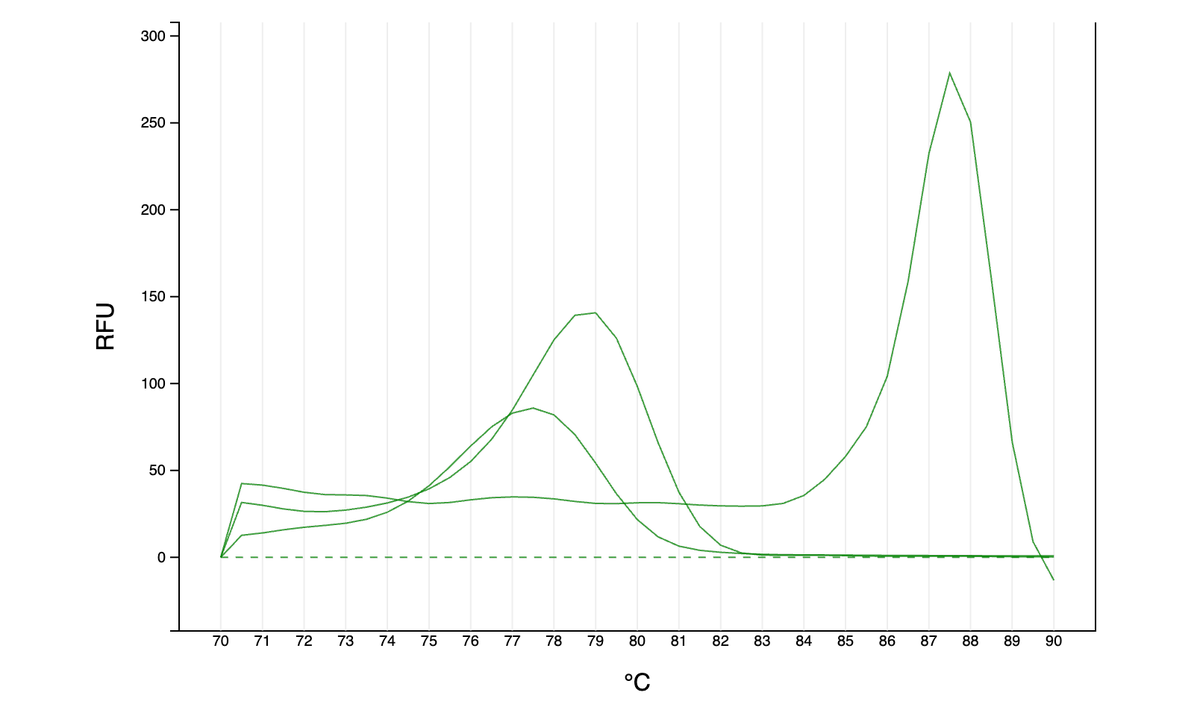
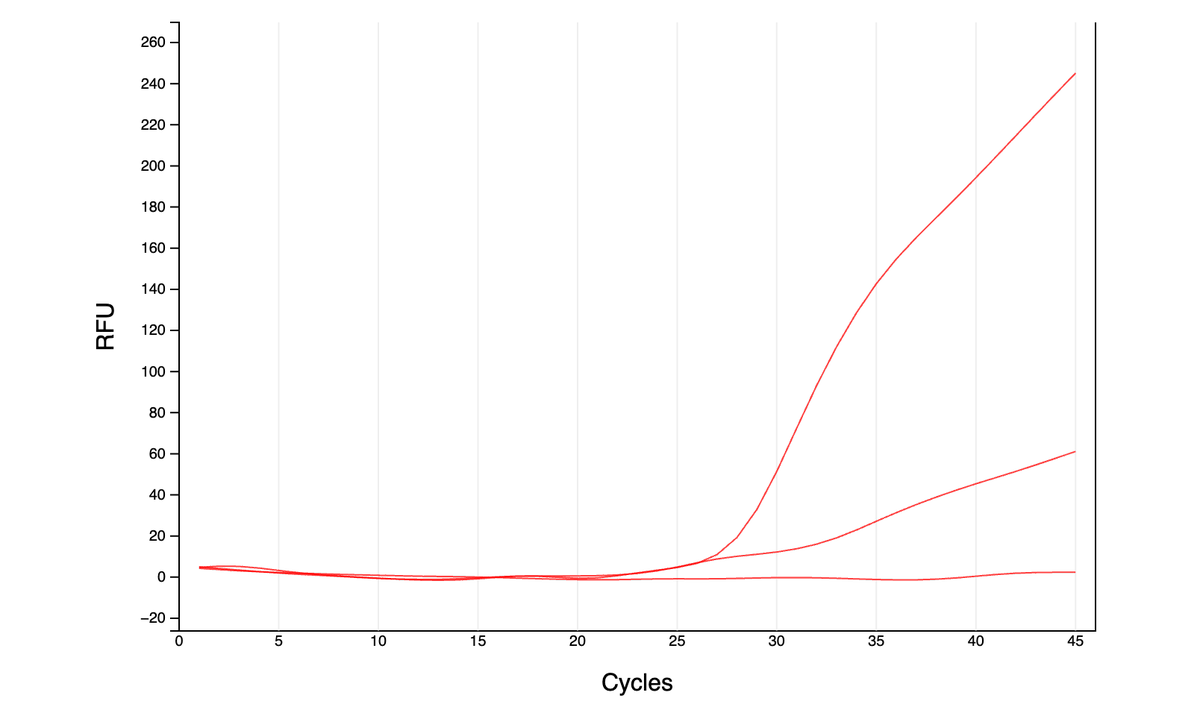
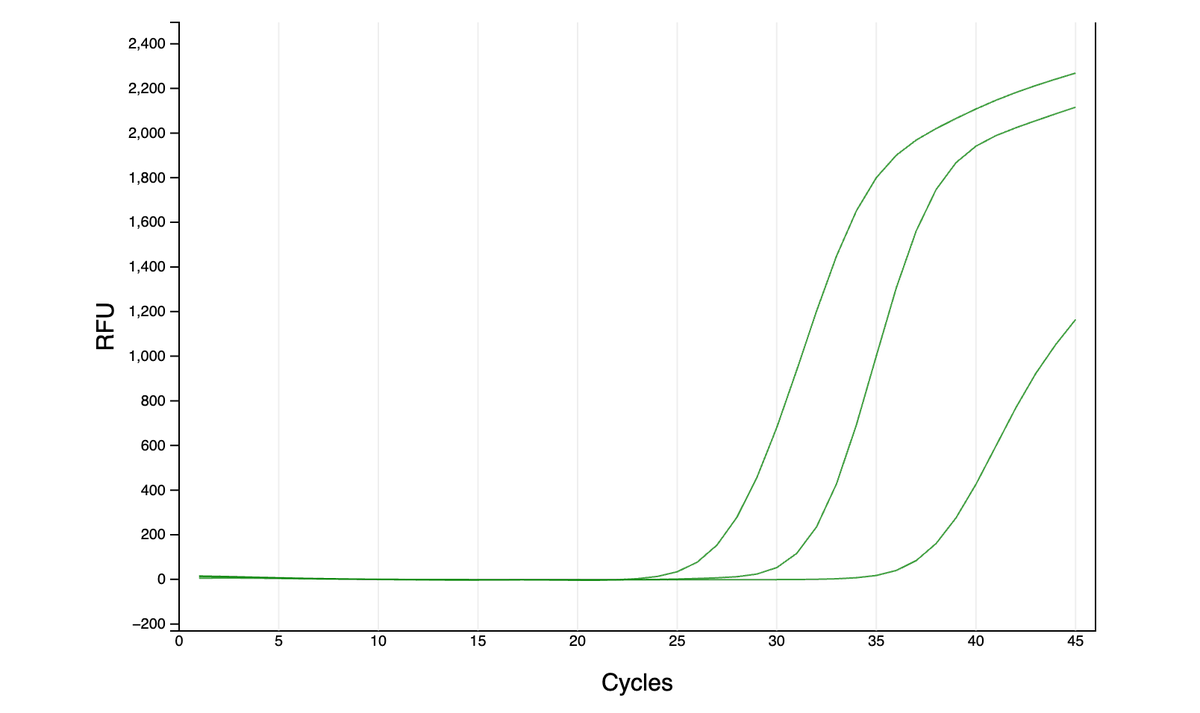
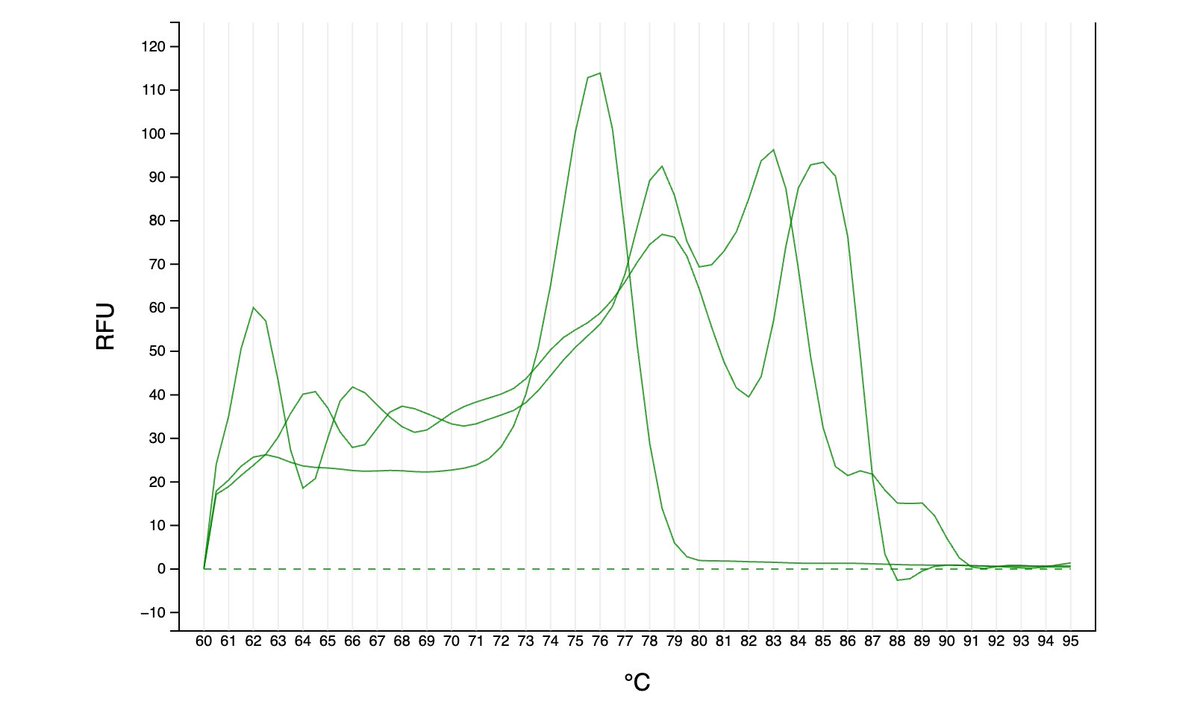
As far as I can tell from the literature, qPCR is generally done either with dye (quantify all amplified DNA and it’s melting temp - green graphs here) or with more precise TaqMan probes (detect a specific region of amplified DNA - red graph). However, …
Am I the only one who finds using both simultaneously useful? Is it just that I’m terrible at PCR and real scientists don’t need to troubleshoot to the same degree? Or maybe they troubleshoot as much as I do but never write about it? 🙂
I started this because I ran out of probe master mix and decided to try using the 2-year old (and long-expired) dye master mix I still had in my freezer before ordering more. 😁